Q&A
This Q&A has been prepared to answer questions about PHederation and related topics.
Real-world data (RWD) is data that is routinely collected from a variety of sources such as electronic health records (EHRs), product and disease registries, and others. The analysis of these data then results in real-world evidence (RWE).
Examples of the type of analyses that can be performed on the PHederation platform include:
Healthcare Professionals and Patient Community
- Investigate the use of risk assessment at diagnosis/baseline and during follow-up in patients with pulmonary hypertension (PH)
- Evidence of disease outcomes from different treatment strategies
- Identify factors increasing persistency and adherence to PH medication
- Support development of early diagnostic algorithms in Electronic Health Records using advanced analytics
Improve R&D / regulatory discussions
- Serve as external controls in clinical trials
- Contextualize clinical trials through inclusion of RWE-based analyses into the submission dossier
- Regulatory scientific advice to plan for RWE-based analyses available at time of submission to provide context around clinical trials
Improve access to treatment / reimbursement
- Demonstrate the burden of disease using national data advocating for access to treatment
Data dashboards will describe data assets content on PHederation for registered members. The original data source is locally transformed to a common data model structure. Subject level data is not shared with PHederation.
For the conduct of studies, the actual data analyses are carried out locally and results will be only shared with the study team. Published results will be available in the PHederation study catalogue.
For the exchange and retention of results, PHederation ensures data protection using the highest security standards.
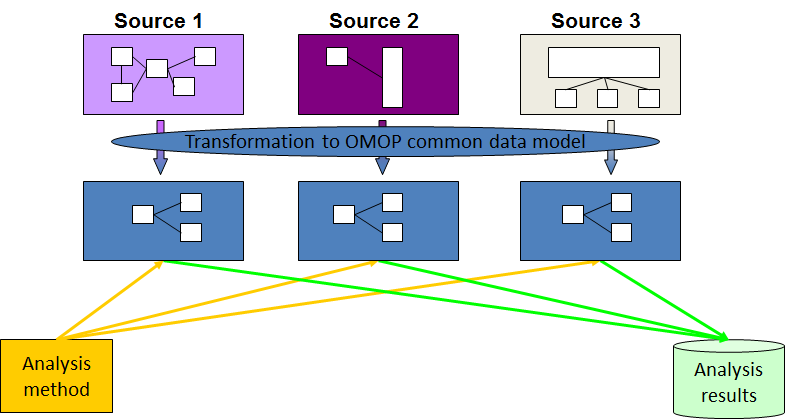
Comparing patients’ records from around the globe can be challenging. PHederation uses the OMOP common data model to map all data to one standard, using a robust process.
The OMOP common data model allows for the systematic analysis of disparate observational databases. The concept behind this approach is first to transform data contained within those databases into a common format (data model) as well as a common representation (terminologies, vocabularies, coding schemes); then to perform systematic analyses using a library of standard analytic routines that have been written based on the common format.
Pre-requisites for each data asset
Each data asset need to agree on transforming their data into the "Observational Medical Outcomes Partnership" (OMOP) "Common Data Model" (CDM)
For more information:
https://www.ohdsi.org/data-standardization/the-common-data-model/
The PHederation platform is designed with patient privacy in mind. All personal data remains local with the participating party. To participate in PHederation network, each party guarantees that its data provenance complies with relevant Data Protection Legislation and ensures a legal basis of using such personal data for secondary scientific research purposes.
Feasibility studies can be done using dashboards where anonymization techniques are employed to minimize the risk of a patient from being singled out. Only aggregated and anonymized results are exchanged within the central PHederation portal.
Results are always transferred via a secure connection. On the portal itself, results are encrypted and only accessible to the researchers who participated in the study. Applicable data protection rules and regulations are always adhered to with appropriate privacy safeguards in place.
Didn’t find the answer to your question here? Feel free to contact us via e-mail.
We will use your information in accordance with our Privacy Policy.
REFERENCE: 19016998 | DATE OF PREPARATION: DECEMBER 2019
